Research
Reaction Methodology/Synthesis Strategy
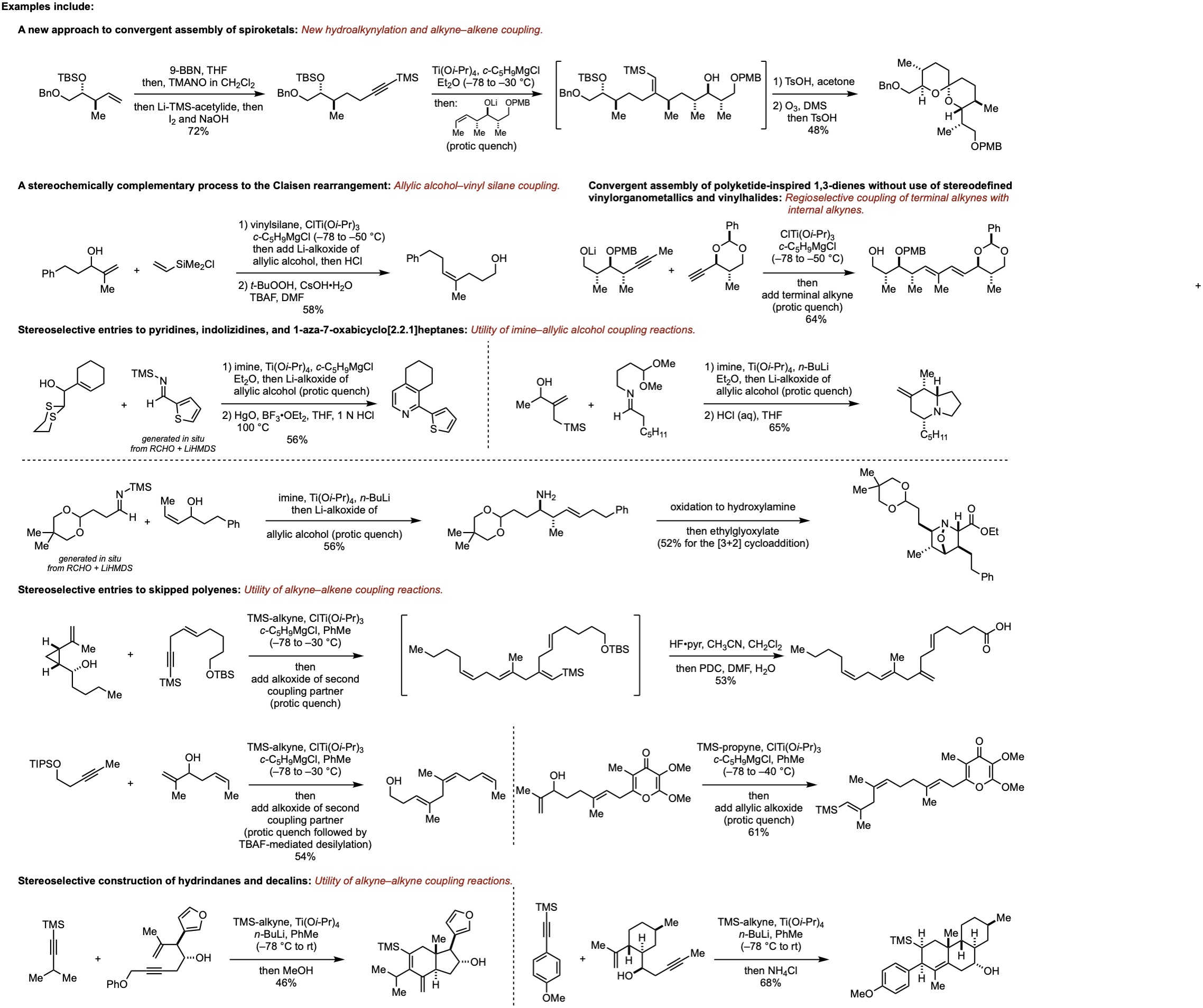
We routinely conceive of, and establish, new reactions to facilitate complex molecule synthesis. We pursue this course of study to define science that can provide for fundamentally unique synthesis strategies for de novo construction of natural products, with the aspirational goal that our technology may be used to enable future natural product-inspired drug discovery research programs. It is always of great interest to us that our chemical methods afford novel retrosynthetic relationships for organic synthesis, and that they address structures that are otherwise quite challenging/costly to prepare using existing technology. We believe that contributions like these can have broad impact in pharmaceutical science, with potential to shape the future of medicinal chemistry and drug discovery.
While there are many types of reactions that we have secured, having established >40 unique stereoselective transformations to date, we are particularly concerned with advancing reaction methods and synthesis strategies that solve challenging problems associated with stereo- and regiocontrol in carbon–carbon bond-forming reactions. To date, our developed reactions provide for new approaches to construct heterocycles, polyketides, and terpenoids. Please see our publications listed on this site for more information (this tab includes graphical abstracts for most publications).
Natural Product Synthesis
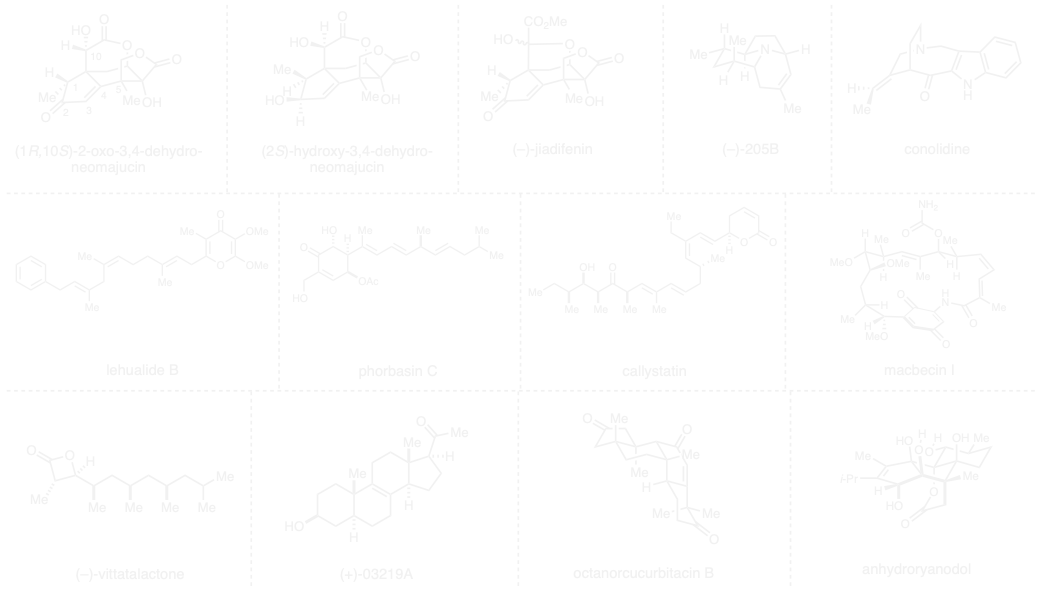
In concert with our efforts directed at new synthetic methods, we are actively engaged in research aimed at demonstrating the utility of these methods in the synthesis of biologically important natural products. These pursuits often do not end with the completed laboratory synthesis of a rare natural product. Rather, the chemical solution defined serves as a scientific foundation to enable collaborative
Examples of natural product targets and families that have been of interest (not meant to be comprehensive, but rather to show the diversity of targets of interest): ryanadol, pentacyclic triterpenoids, limonoids, cardenolides, bufadienolides, euphol, tirucallol, lanosterol, octanorcucurbitacin B, marine pregnene (+)-03219A, alkaloid 205B, corialactone D, pectenotoxin 2, (–)-jiadifenin and other neurotrophic seco-prezizaane sesquiterpenoids, macbecin, callystatin A, dictyostatin, soraphen A, lehualide B, vittatalactone, conolidine, and phorbasin C.
The graphic below shows some of the structures of the natural products that we have synthesized:
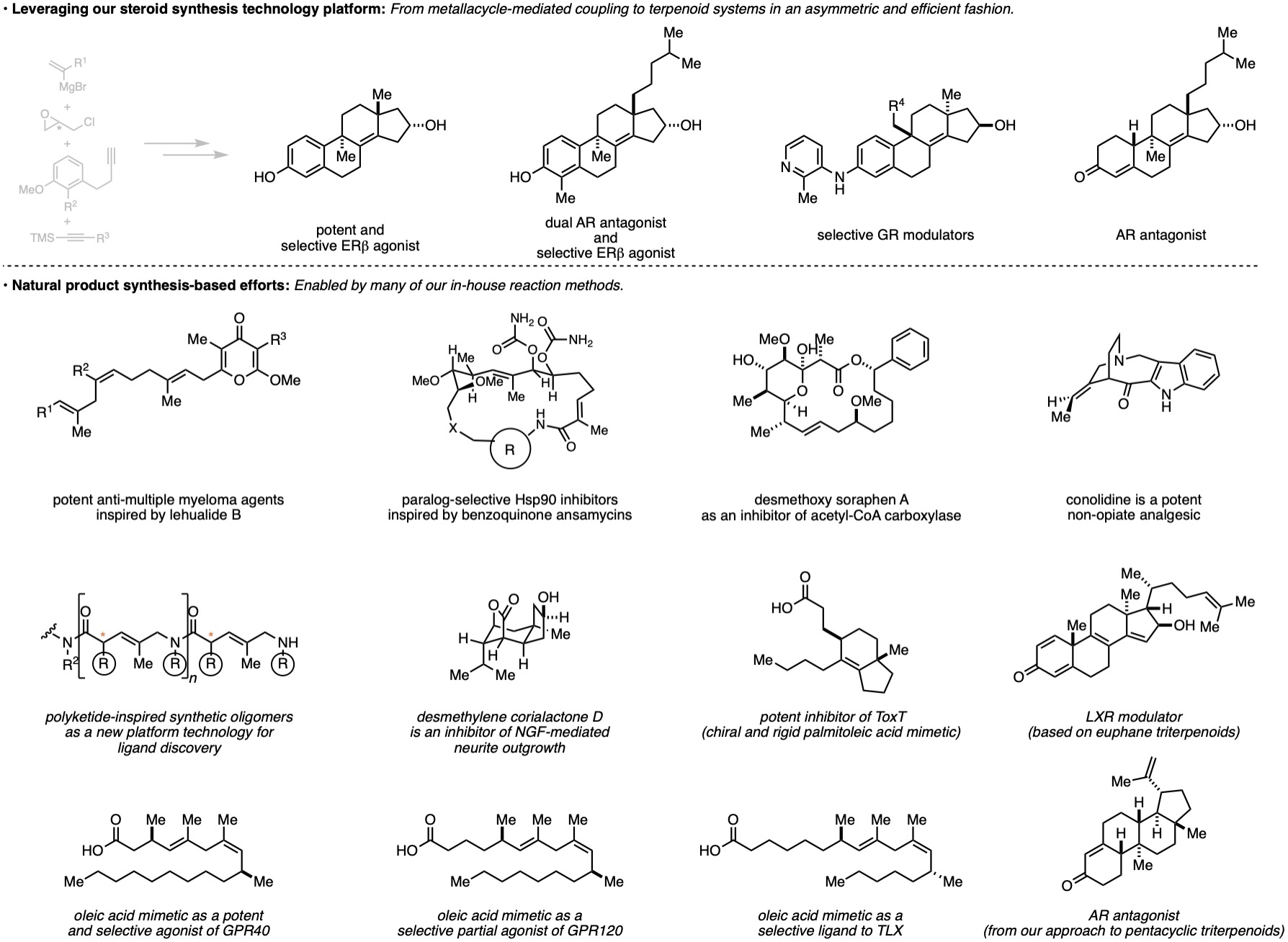
Natural Product-Inspired Function-Oriented Synthesis
Our approach to the design, discovery, and development of new reaction methods and strategies for complex molecule synthesis has the potential of providing enabling technologies suitable to drive efforts targeting the discovery of new biologically/therapeutically relevant small molecules. We are actively involved in such programs, and focus on developing the basic organic chemistry necessary to drive such pursuits. To date, these efforts take the form of targeting synthetic variants of natural products with potential therapeutic value, as well as an unbiased diversity-oriented approach, where common natural product motifs provide inspiration for the synthesis of large and diverse small molecule compound collections from which novel biological probes and/or therapeutics can be discovered. In each case, our efforts are soundly grounded in organic synthesis while our goals target interdisciplinary and collaborative pursuits at the interface between chemistry, biology and medicine.
Examples of projects that have been conducted in this area include: (1) oleic acid mimetics as agonists of TLX and GPR40, (2) palmitoleic acid mimetics as inhibitors of ToxT, (3) a network of synthetic transformations to revolutionize the search for medicinally relevant steroids (recent applications have resulted in the identification of a supremely potent and selective agonist of ER-beta, a collection of dissociated glucocorticoids, and a dual ER-beta agonist and AR antagonist, (4) a des-methoxy soraphen A as an inhibitor of acetyl-CoA carboxylase, (5) anti-multiple myeloma agents inspired by lehualide B, (6) paralog-selective Hsp90 inhibitor based on the benzoquinone ansamycins, (7) the identification/discovery of the potent non-opioid analgesic conolidine, and (8) a synthetic oligomerization inspired by polyketide structures that results in chiral oligomers of pentenoic amides (a large compound class that can be used in one bead-one compound- or DNA-encoded library technology).